|
Green hydrogen is widely regarded as a key enabler of the global energy transition, as it provides an essential pathway for the rapid decarbonization of industries that cannot be directly electrified. These carbon intensive industries include aviation, shipping, steel, cement, fertilizer, chemical production and many more - which together, account for approximately 30% of global CO2 emissions. Whilst hydrogen has been used for over a century, its means of production must make way for newer and more sustainable methods.
About hydrogen?
Hydrogen is one of the most abundant elements on earth, and already plays a crucial role in today's energy landscape. According to the International Energy Agency (IEA), hydrogen's use tripled from 1975 to 2018. Whilst hydrogen is a dense and flexible energy carrier, when produced using fossil fuels, it emits a significant volume of CO2 emissions.
What is green hydrogen and how is it produced?
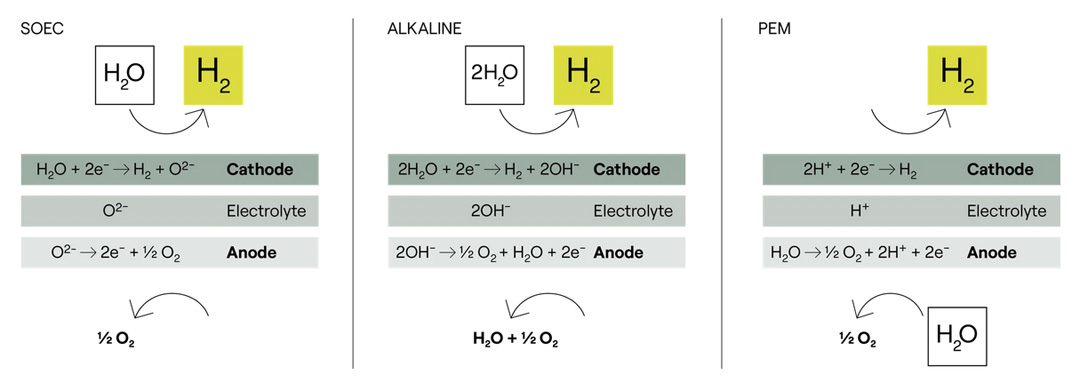
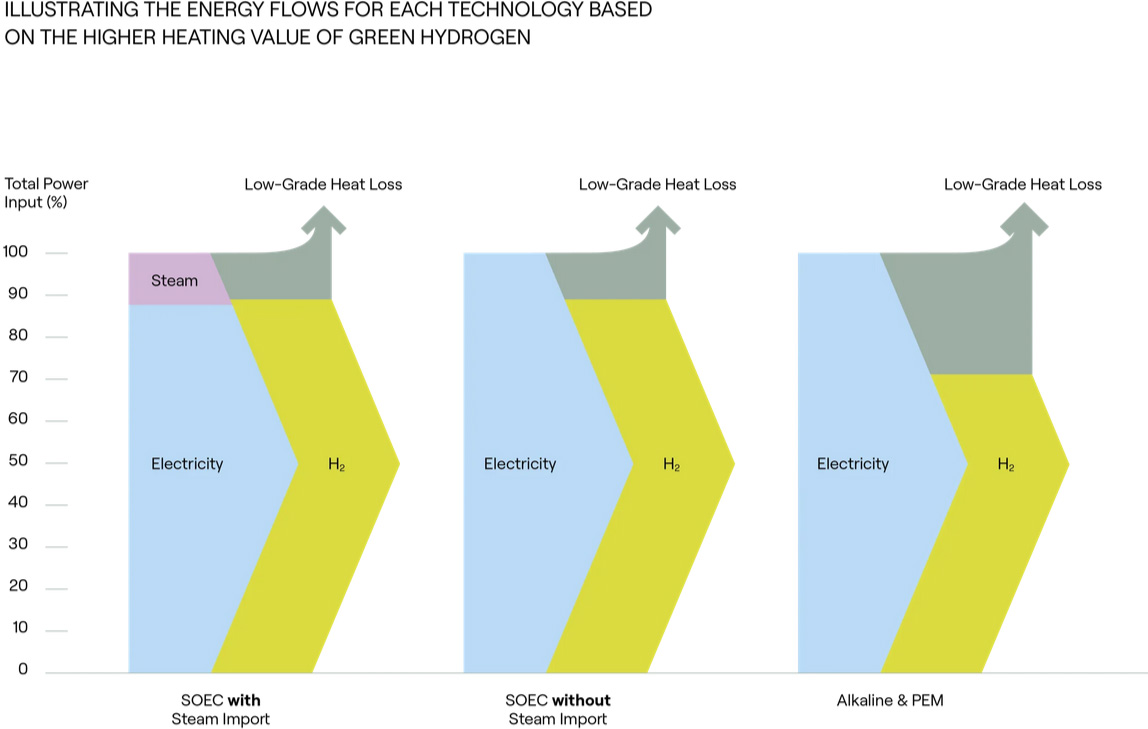
Green hydrogen is the product of electrolysis, a process that separates water into its two elemental components: hydrogen and oxygen. Electrolysis requires electrical current, which is supplied by renewable energy sources such as wind, solar, hydroelectric or nuclear power. This production method ensures that the hydrogen is generated with the lowest possible CO2 emissions.
Green hydrogen's role in the energy transition?
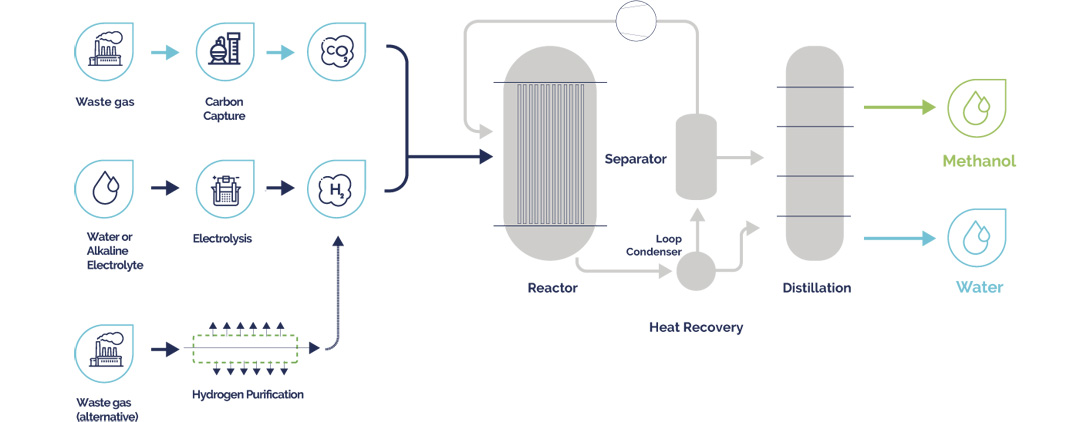
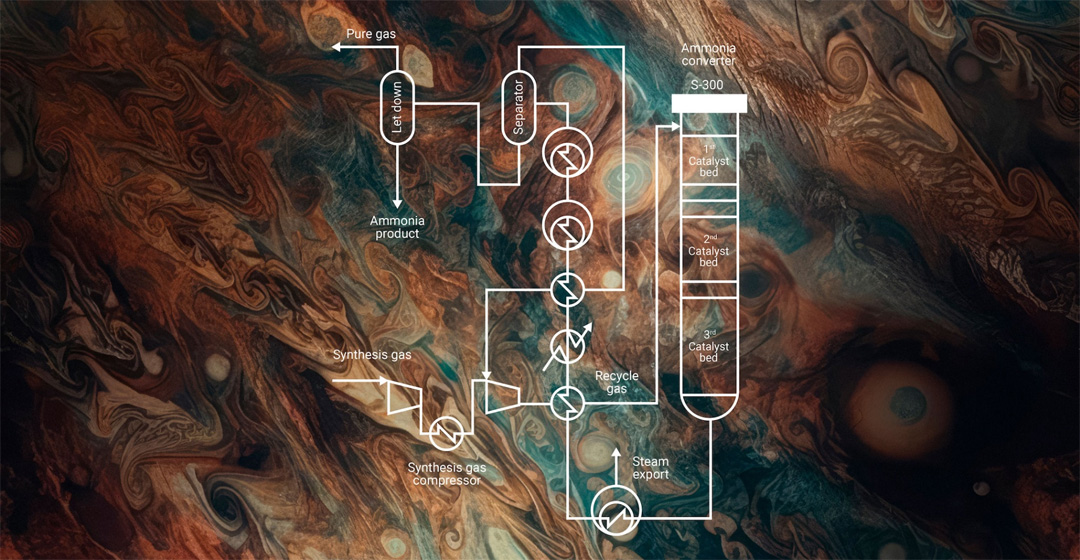
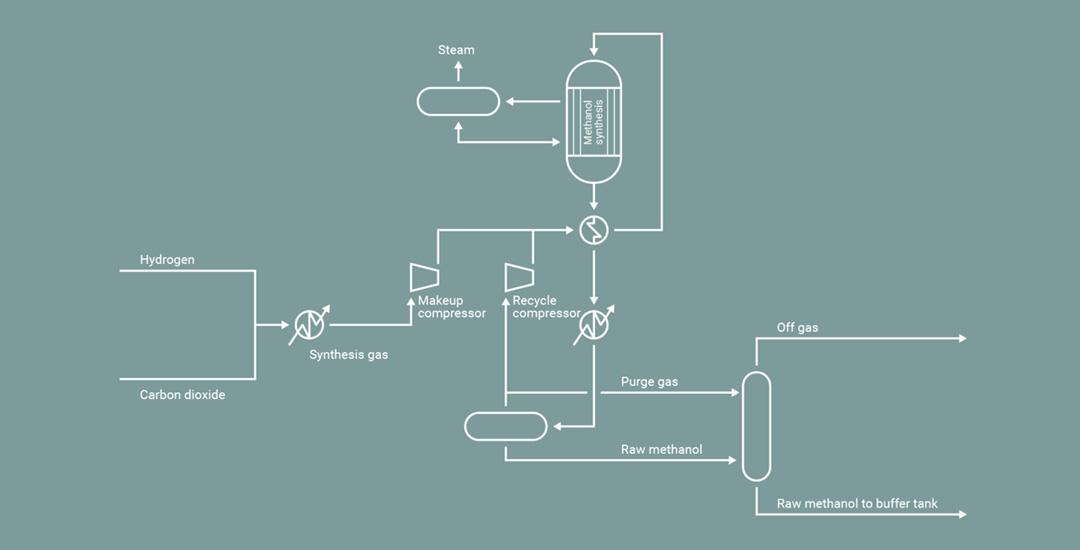
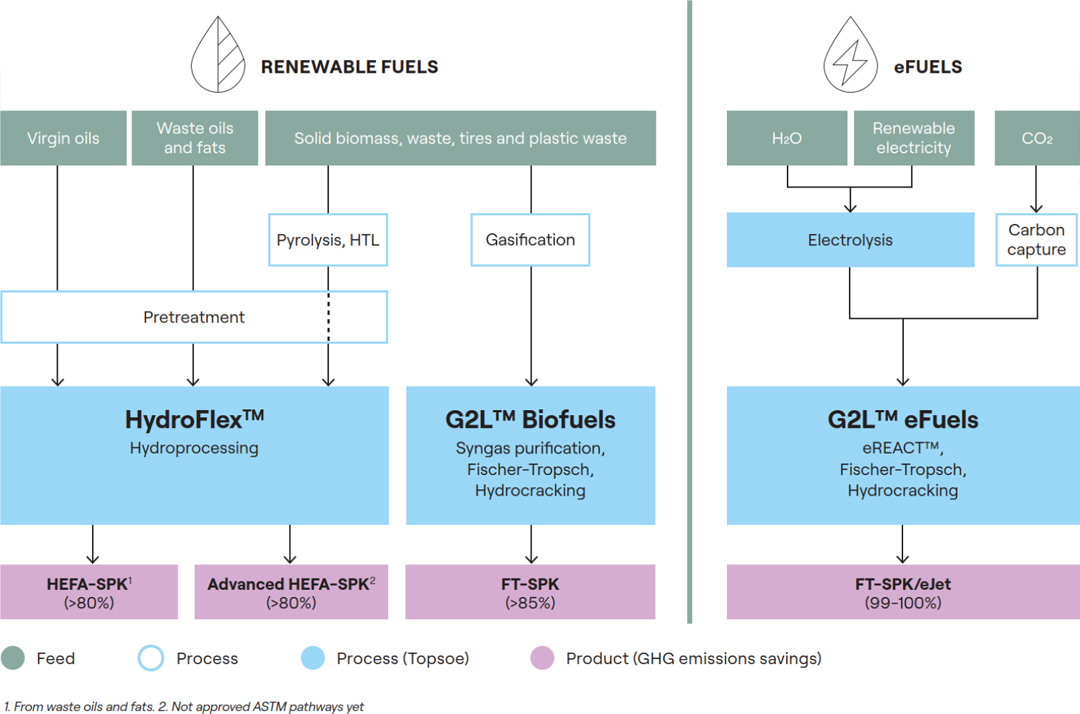
Green hydrogen will transform the energy landscape by providing a sustainable and scalable solution to meet both current and future energy needs. From its use as direct replacement for the fossil fuels currently powering heavy transport and industrial processes, green hydrogen can be easily synthesized into a range of eFuel derivatives. From eMethanol and green ammonia, to its role in the production of electrified Sustainable Aviation Fuel (eSAF) - green hydrogen is a true lynchpin of the energy transition.
|